Journal of the InterAmerican Medical and Health Assn.
January-April 1992, Vol.1, No.1
IMMUNE IMPAIRMENT AND THE HYPOTHESIS OF THE "ACQUIRED IMMUNE DEFICIENCY CYCLE"Dr. Maurizio Luca Moretti
ABSTRACTBased on the fact that infection, malnutrition, and immunosuppression are well-known causes of immune impairment, their effects in the host are being reviewed. The findings suggest that infection, malnutrition, and immunosuppression can interact as synergistic causes of immune impairment, and could also be components of a cycle capable of inducing immune impairment. Based on these findings, it has been hypothesized that the "Acquired Immune Deficiency Cycle" (AIDC) is a physiological cycle that could be a primary cause of temporary, chronic, or congenital immune impairment.
INTRODUCTION
Various degrees of immune impairment have been found among HIV-seronegative individuals at high risk for the Acquired Immune Deficiency Syndrome (AIDS); namely, HIV-seronegative hemophiliacs (1-13), HIVseronegative homosexual and bisexual males (14-26), HIV-seronegative intravenous drug users (IVDUs) and their infants (22,27-31), and HIV-seronegative heterosexuals of developing countries (32). These findings strongly suggest that the subjects' immune impairment is a result of factor(s) other(s) than Human Immunodeficiency Virus (HIV) infection (33,34) .
Three major adverse factors have been shown to be common among individuals at high risk for AIDS; namely, increased risk of infection (1,3,15,20-22,25,30,32,35-73), increased risk of malnutrition (31-34,72,74-98), and increased risk of immunosuppression (1,7,10,12,18,31-34,38-40,82-89,99-108). This is not surprising since a number of habitual practices by individuals at high risk for AIDS are potential causes of infections (32-34,64-73,84-98,109-133), malnutrition (109-111,134-156), and immunosuppression (1,7,10,12,18,31-34,38-40,82-89,99-108).
Therefore, it is important to realize that infection (35-37,157-211), malnutrition (212-248), and immunosuppression (1,7,10,12,18, 38-40,99-108) are major causes of immune impairment (1,7,10,12,18,24,35-40,99-108,157-248).
When the effects of infection, malnutrition, and immunosuppression are further analyzed, it can be observed that they can interact as synergistic causes of immune impairment.
INFECTION, MALNUTRITION, AND IMMUNOSUPPRESSION AS SYNERGISTIC CAUSES OF IMMUNE IMPAIRMENT
I. INFECTION
Infection can cause both immunosuppression (35-37,157-204) and malnutrition (109-111,134-151). As a result, the induced malnutrition can cause immunosuppression or increase a current immunosuppression (126,127,212-233,235-253). The induced immunosuppression can cause both increased frequency and severity of infection(s), and out-break of opportunistic disease(s) (109,212,254-261) .
II. MALNUTRITION
Malnutrition can cause immunosuppression (126,127,212-233,235-253). As a result, the induced immunosuppression can cause both increased frequency and severity of infection(s) and out break of opportunistic disease(s) (109,212,254-261). The induced infection can cause both immunosuppression (35-37,157-204) and malnutrition (109-111,134-151) .
III. IMMUNOSUPPRESSION
Immunosuppression can cause both increased frequency and severity of infection(s) and out-break of opportunistic disease(s) (109,212,254-261). As a result, the induced infection can cause both immunosuppression (35-37,157-204) and malnutrition (109-111,134-151). It seems reasonable, therefore, to conclude that infection, malnutrition and immunosuppression not only can interact as synergistic causes of immune impairment, but also could be components of a physiological cycle capable of inducing immune impairment in the host. Based on these findings, the following is hypothesized about the "Acquired Immune Deficiency Cycle" (AIDC).
HYPOTHESIS
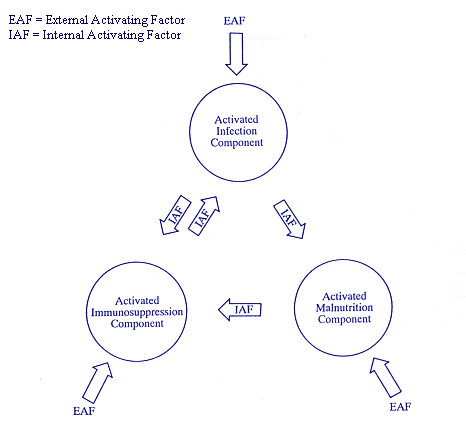
The Acquired Immune Deficiency Cycle
(Figure I)
AIDC Characteristics
Structure. The Acquired Immune Deficiency Cycle (AIDC) comprises three synergistic components: infection, malnutrition, and immunosuppression.
External Activation. AIDC can be activated through its components by an external activating factor (EAF), specifically a potential cause of infection, malnutrition, or immunosuppression .
Internal Activation. AIDC can be self-activated through its components by an internal activating factor (IAF), specifically an AIDC component currently activated by a condition of infection, malnutrition, or immunosuppression.
Inactivation. AIDC can become inactive when both EAFs and IAFs are neutralized. Then the organism itself, under normal physiological and psychological conditions, should correct the immune impairment caused by AIDC's activation.
Adverse effects. Depending on the frequency and intensity of its activation, AIDC can be a primary cause of a temporary, chronic, or congenital immune impairment in the host.
The following explanation of AIDC's mechanism may offer approaches to better understand how AIDC could be activated through specific activating factors (AFs), which are common among individuals at high risk for AIDS (1,7,10,12,18,31-34,38-40,64-73,82-156). However, it is imperative to consider that AIDC's adverse effects on the immune system would not be limited only to individuals at high risk for AIDS.
I. AIDC's INFECTION COMPONENT ACTIVATION
AIDC could be activated through its infection component by the following AFs, which are common among individuals at high risk for AIDS, because many of their habitual practices are potential causes of infections (32-34,64-73,84-98,109-133). Consequently, individuals at high risk for AIDS are also at increased risk for infections (1,3,15,20-22,25,30,32,35-73). The following illustrate specific examples of potential causes of either symptomatic or asymptomatic infection.
(A) Intravenous drug addiction is a potential cause of infections. These can be caused by the following: (a) The injection of contaminated "street drugs" (84,85,90) into the bloodstream. (b) The use of a contaminated needle or syringe (124,125) .
(B) The sexual practice of anal intercourse is a potential cause of infections. These can be caused by the following: (a) The contact of genital mucosa with intestinal microorganisms contained in fecal material (91-98) (Table I) during this practice. (b) The contact of genital mucosa with a causative microorganism(s) of anorectal sexually transmitted diseases (114-123) (Table II).
(C) Vaginal intercourse, fellatio, or cunnilingus, if at least one partner is at high risk for AIDS, are potential causes of infections. These can be caused by the following: (a) Heterosexual or orogenital contact with a partner who is at high risk for AIDS (1,3,15,20-22,25,30,32,35-73). (b) Heterosexual orogenital transmission of causative microorganisms of sexually transmitted diseases (114-118).
(D) The practice of "anilingus" is a potential cause of infections. These can be caused by contact of oral mucosa with intestinal microorganisms contained in fecal material (91-98) (Table I).
(E) Erotic activity, such as fist fornication (commonly known as "fisting") is a potential cause of infections. This practice consists of receiving into the rectal cavity the insertion of the sexual partner's hand, up to the wrist and even the forearm (126,262). These infections can be caused by intestinal microorganisms contained in one's own fecal material (91-98) (Table I) through abrasion(s) and/or laceration(s) of the anus and/or rectum resulting from this practice (127).
(F) Erotic activity such as the insertion of object(s) into the anus and rectum (128,129), is a potential cause of infection(s) . These are caused by intestinal microorganisms contained in one's own fecal material (91-98) (Table I) through tissue laceration of the anus and/or the rectum resulting from this practice (129,130).
Table I INFECTIONS TRANSMITTED BY CONTACT WITH FECAL MATERIAL Causative organism DISEASE Campylobacter GASTROENTERITIS AND OTHERS (183) Hepatitis A virus HEPATITIS A (178) Shigella SHIGELLOSIS (179) Salmonella Typhi TYPHOID FEVER (180) Entamoeba histolytic AMEBIASIS (181) Cryptosporidia CRYPTOSPORIDIOSIS (182) Giardia lamblia GIARDIASIS (184) Enterobius vermicularis PINWORM INFESTATION (185)
Table II SEXUALLY TRANSMITTED DISEASES Causative organism Sexual contact Disease Neisseria gonorrhoea Heterosexual
Anorectal
OrogenitalGONORRHEA (209)
RECTAL GONORRHEA (209)Treponema pallidum Heterosexual
Anorectal
OrogenitalSYPHILIS (210)
RECTAL SYPHILIS (210)Chlamydia trachomatis Heterosexual
AnorectalLYMPHOGRANULOMA (211)
VENEREUM (211)Herpes simplex virus Heterosexual
AnorectalGENITAL HERPES (212)
ANOGENITAL HERPES (212)Human papilloma virus Heterosexual
AnorectalGENITAL WARTS (213) Gonococci Anal-receptive
intercoursePROCTITIS (214) Human papilloma virus Anal-receptive
intercoursePROCTITIS (215) Herpes simplex virus Anal-receptive
intercoursePROCTITIS (216) Syphilis Anal-receptive
intercoursePROCTITIS (217) Chlamydia trachomatis Anal-receptive
intercoursePROCTITIS (218) (G) Erotic activity such as the use of enemas (klismaphilia, commonly known as "water sports") for erotic or erotic-hygienic purposes before and/or after anal intercourse, (86-89) is a potential cause of infections. Improper insertion of the nozzle, during this practice, can cause laceration of the anus and/or rectal mucosa (131,132), or possible perforation of the rectal wall (228) allowing infections by intestinal microorganisms contained in one's own fecal material (91-98) (Table I).
(H) The use of commercial clotting factors is a potential cause of infections. These are caused by pathogenic microorganism(s) disseminated in the plasma pools used to elaborate commercial clotting factors (112). These pathogenic microorganisms are still undetectable during blood screening due to limitations of current technology (113) .
(I) Pregnancy under adverse conditions such as current infectious disease(s), is a potential cause of vertical transmission of infection(s). Vertical transmission of infections is common among the fetuses of mothers at high risk for AIDS (64,67).
(J) Infections are common among the populations of developing countries (32,68-73,109-111) in which infections and their associated immune impairment (32,35 37,157-211) are main causes of the high mortality rates (263) .
As a consequence of one of the previous AFs, the AIDC's activated infection component could act as an IAF of both immunosuppression (35-37,157-204) and malnutrition (109-111,134-151) AIDC's components. As a result, the induced immunosuppression could act as IAF to cause both increased frequency and severity of infection(s) and out-break of opportunistic disease(s) (109,212,254-261). Thus, the induced malnutrition could act as IAF to cause immunosuppression or increase a current one (126,127,212-233,235-253).
II. AIDC's MALNUTRITION COMPONENT ACTIVATION
AIDC could be activated through its malnutrition component by the following AFs, which are common among individuals at high risk for AIDS, because many potential causes of malnutrition (109-111,134-156) are common among individuals at high risk for AIDS (1,3,15,20-22,25,30,32-81,109-111,152,156, 263-265). Consequently, individuals at high risk for AIDS have increased risk for malnutrition (31-34,72,74-98). The following illustrate some specific examples.
(A) Infection(s) cause malnutrition (109111,134-151) and infections are common among individuals at high risk for AIDS (1,3,15,20 22,25,30,32,35-73).
(B) Diarrheal diseases are causes of malnutrition (153-155) and diarrhea! diseases are common among individuals at high risk for AIDS (32,72,74-81). This is because most of the infections caused by intestinal microorganisms contained in fecal material (91-98) (Table I) also can cause chronic diarrhea, which is a cause of malabsorption and consequentially malnutrition (153-155).
(C) Bleeding, a potential cause of malnutrition, is common among hemophiliacs (152) and homosexual men (rectal bleeding) (156) .
(D) Malnutrition is common among the populations of developing countries (263-265) in which malnutrition and its associated immune impairment (126,127,212-233,235 -253) are main causes of a high mortality rates (263) .
As a consequence of one of the previous AFs, the AIDC's activated malnutrition component could act as an IAF of immunosuppression (126,127,212-233,235-253) AIDC's component. As a result, the induced immunosuppression could act as IAF to cause both increased frequency and severity of infection(s) and out-break of opportunistic disease(s) (109,212,254-261).
III. AIDC's IMMUNOSUPPRESSION COMPONENT ACTIVATION
AIDC could be activated through its immunosuppression component by the following AFs, which are common among individuals at high risk for AIDS, because many of their habitual practices are potential causes of immunosuppression (1,7,10,12,18,31-34,38-40,82-89,99-108). The following illustrate some specific examples.
(A) Injection of "foreign substances" contained in " street drugs" (84,85), or heroin itself (even if 100% pure) (31,82,83) into the bloodstream has immune suppressive effects (31,82-85).
(B) Exposure of rectal mucosa to seminal plasma has immune suppressive effects (18, 37,40,102-108).
(C) Use of an enema with up to four gallons of water for erotic purposes (klismaphilia, commonly known as "water sports" ), or erotic-hygienic purposes before and/or after anal intercourse (86-89) has immune suppressive effects. The immune suppressive effects are caused by an abnormal elimination of antibodies from the intestinal mucosa due to the mechanical action of the water used during the enema.
(D) Use of commercial clotting factors has immune suppressive effects. These are caused by chronic exposure to multiple protein antigens and immune complexes contained in pooled-blood products derived from thousands of donors (1,7,10,12,38,99-101).
(E) Pregnancy during current immune impairment or current drug abusing practice has immune suppressive effects on the fetus (31).
(F) Immunosuppression (31) and its associated immune impairment (1,7,10,12,18, 38-40,99-108) are common among the populations of developing countries (31,32) where these are the predominate causes of high mortality rates (263).
As a consequence of one of the previous AFs, the AIDC's activated immunosuppression component could act as IAF of infection (109,212,254-261) AIDC's component, through both increased frequency and severity of infection(s) and out-break of opportunistic disease(s). As a result, the induced infection could act as a IAF to cause both immunosuppression (35-37,157-204) and malnutrition (109-111,134-151).
PROSPECT
Based on a unanimous consensus of the importance to avoid immune impairment, we are confident that the understanding and control of AIDC's activation could be an invaluable tool in the prevention and treatment of its associated immune impairment. The prevention of AIDC's activation should be achieved through the avoidance of AIDC's AFs, specifically potential causes of infection, malnutrition, and immunosuppression. The treatment of AIDC's associated immune impairment should be achieved through AIDC's inactivation by a multi-disciplinary effort to control the patient's current infection(s), malnutrition, and immunosuppression, thus avoiding their consequential immune impairment. If this could be achieved, the high mortality rate associated with immune impairment, through increased risk of infections, should be expected to decrease dramatically.
REFERENCES
1. Sullivan JL, Brewster FE, Brettler DB, et al. Hemophiliac immunodeficiency: influence of exposure to factor VIII concentrate, LAV/HTLV-III, and herpes viruses. J Pediatr 1986; 504-10.
2. Freedma J, Mazaheri R, Read S, Garvey MB, Teitel J. Humoral and cellular immune abnormalities in adult hemophiliacs followed over a 2-year period. Diagn and Clin Immunology 1987; 5:30-40.
3. Shannon BT, Roach N, Cheek-Luten M, Orosz C, Ruymann FB Progressive change in lymphocyte distribution and degree of hypergammaglobulinemia with age in children with hemophilia. J Clin Immunology 1986; (6)2:121-9.
4. Choremi H, Sidiri E, Psarra K, Economidou J, Georgiopoulou P. Immune status of Greek patiens with B-thalassemia major negative for anti-HIV. BLUT 1987; 54:267-73.
5. Matheson DS, Green BJ, Fritzler MJ, Poon MC, Bowen TJ, Hoar DI. Humoral immune response in patients with hemophilia. Clin Immunology and Immunopathology 1987; 4:41-50.
6. Rodeghiero F, Castaman GC, Chisesi T, et al. One year follow-up study of T-cell subsets and incidence of seropositivity for HTLV-I and HTLV-III antibodies in patients treated " on demand " or sporadically with clotting concentrates. Thrombosis and Haemostasis. FK Shattauer Verlag GmbH (Stuttgart) 1985; 54(3):665-8.
7. Madhok R, Gracie A, Lowe GD, et al. Impaired cellmediated immunity in haemophilia in the absence of infection with human immunodeficiency virus. Brit Med J 1986; 293:978-80.
8. McVerry BA, Machin SJ, Galloway MJ, et al. HTLV-III antibody and T-cell subset ratios in haemophiliacs and their spouses. Br J Haem 1986; 63:347-52.
9. Ramsey RB, Palmer EL, McDougal JS, et al. Antibody to lymphoadenopathy-associated virus in haemophiliacs with and without AIDS. Lancet 1984; ii: 397-8.
10. Tsoukas C, Gervais F, Shuster J, Gold P. Association of HTLV-III antibodies and cellular immune status of hemophiliacs. N Eng J Med 1984; 311:1514-5.
11. Cornu F. Raffoux C, Maisonneuve P, Bardin JM, Sultan Y, Colombani J. HLA typing in heavily transfused haemophiliacs with or without antibodies against human immunodeficiency virus (HIV). Tissue Antigens 1987; 29:123-6.
12. Ludlam CA, Tucker J, Steel CM, et al. Human T-lymphotropic virus type III (HTLV-III) infection in seronegative haemophiliacs after transfusion of factor VIII. Lancet 1985; 233-6.
13. Stein SL, Evatt BL, Laurence DN, et al. Immunologic correlates of infection with HTLV-III /LAV in patients with hemophilia. Thromb Haemost 1985; 54:196.
14. Rornfeld H, Vande Stouwe RA, Lange M, Reddy MM, Grieco MH. T-lymphocyte subpopulations in homosexual men. New Engl J Med 1982; 307:729-31.
15. Detels R, Fahey JL, Schwartz K, Greene RS, Visscher BR, Gottlieb MS. Relation between sexual practices and T-cell subsets in homosexually active men. Lancet 1983; 1:609.
16. Gerstoft J, Orskou-Linhardt B, Petersen CS, et al. Antibodies to human T-cell lymphotropic virus type III in promiscuous healthy homosexual men. Relation to immunological and clinical findings. Eur J Clin Invest 1985; 15:290-5.
17. Estevez ME, Ballart IJ, Diez RA, Planes N, Scaglione C, Sen L. Early defect of phagocytic cell function in subjects at risk for Acquired Immmune Deficiency Syndrome. Scand J Immunol 1986; 24:215-21.
18. Collier AC, Meyers JD, Murphy VL, Roberts PL, Getchell JP, Handafield MM. Relationship between antibody to LAV/HTLV-III and the natural course of subalinical cellular immune dysfunction in homosexual men. Sexually Transm Dis 1987; 14(1):1-8.
19. Williford-Pifer LL, Yung-Fun Wang, Chiang TM, Ahokas R, Woods DR, Joyner RE. Borderline Immunodeficiency in male homosexuals: is life-style contributory? Southern Med J 1987; 80(6):687-97.
20. Bergstrom T, Biberfeldt G, Bottiger B, et al. Impaired production of alpha and gamma interferon in asymptomatic homosexual males. Eur J Clin Microbiol 1986; 5(5):523-9.
21. Abrams ID. Lymphoadenopathy syndrome in male homosexuals. Advances of Host Defense Mechanisms. Gallin JI, Fauci A. eds, Raven Press , New York, 1985; 5:75-96.
22. Sirianni MC, Rossi P, Scarpati B, et al. Immunological and virological investigation in patients with lymphoadenopathy syndrome and in a population at risk for acquired immunodeficiency syndrome (AIDS). J Clin Immunol 1985; 5:261-8.
23. Reinherz EL, Kung PC, Golstein G, Schlossman SF. Separation of functional subsets of human T-cell by monoclonal antibody. Proc Natl Acad Scien 1979; 76:4061-5.
24. Reinherz EL, Xung PC, Goldstein G, Schlossman SF. A monoclonal antibody reactive with the human cytotoxic/suppressor T-cell subset previously defined by a heteroantiserum termed TH2. J Immunol 1980; 124:1301-7.
25. Sirianni MC, Testi R, Bonomo G, Aiuti F. Lack of specific cell-mediated immunity to cytomegalovirus in people at risk for AIDS. Aids Res 1984; 99-105.
26. Drew WL. Is cytomegalovirus a cofactor in the pathogenesis of AIDS and Xaposi's sarcoma? The Mount Sinai J Med 1986; 53:622-6.
27. Tirelli U, Vaccher E, Carbone A, et al. Persistent generalized lymphadenopathy: clinical characteristics of a lymphadenopathy syndrome in intravenous drug abusers. Aids Res 1986; 2(3):227-30.
28. Poli G, Bottazzi B, Acero R, et al. Monocyte function in intravenous drug abusers with lymphoadenopathy syndrome and in patients with the acquired immunodeficiency syndrome: selective impairment of chemotaxis. Clin exp Immunol 1985; 62:136-42.
29. Barcellini W, Meroni PL, Lazzarin A, et al. Persistent generalized lymphadenopathy in drug addicts: immunological studies. Boll Ist Sieroter Milan 1986; 65(4):269-76.
30. De Paoli P, Reitano M, Battistin S, et al. Immunological abnormalities in intravenous drug abusers and relationship to the prolonged generalized lymphadenopathy syndrome in Italy . Clin Exp Immunol 1986; 64(3):451-6.
31. Culver XW, Ammann AJ, Partridge JC, Wong DF, Wara DW, Cowan MJ. Lymphocyte abnormalities in infants born to drug-abusing mothers. J Pediatr 1987; 230-5.
32. Quinn TC, Piot P, McCormick JB, et al. Serologic and immunologic studies in patients with AIDS in North America and Africa. JAMA 1987; 257(19):2617-21.
33. Luca-Moretti M. The Acquired Immune Deficiency Cycle: cause or principal co-factor of AIDS? Presented at the Vth International Conference on AIDS, Montreal, Ouebec, Canada, June 4-9, 1989.
34. Luca-Moretti M. Is AIDS prevention effective? Int J Immunopath & Pharm 1990; 3:191-212
35. Hirsch MS, Felsenstein D. Cytomegalovirus-induced immunosuppression. Ann New York Acad Scien 1984; 437:8-15.
36. Rubinstein A, Sicklick M, Gupta A, et al. Acquired immunodeficiency with reversed T4/T8 ratios in infants born to promiscuous and drug-addicted mothers. JAMA 1983; 249:2350-56.
37. Diamantstein T, X103 M, Gold D, Hahn H. Interaction between Entamoeba Histolytica and the immune system. J Immunol 1981; 126:2084-6.
38. Goldsmith JC, Moseley PL, Monick M, McCormick JJ, Walker DY, Hunninghake GW. Immunologic profiles of adults with congenital bleeding disorders. Aids Res 1984; 1(3):163-79.
39. Shearer G. Acquired immune deficiency syndrome (AIDS): a consequence of allogeneic Ia-antigen recognition. Immunol Today 1984; 4:181.
40. Sonnabend JA, Witkin SS, Portilo DT. A multifactorial model for the development of AIDS in homosexual men. Ann New York Acad Scien 1984; 837:177-83.
41. Moffat EH, Bloom AL, Jones J, Matthews N, Newcombe RG. A study of cell-mediated and humoral immunity in haemophilia and related disorders. Br J Haematol 1985; 61:157-67.
42. Enck RE, Betts RF, Brown MR, Miller G. Viral serology: hepatitis B virus, cytomegalovirus, Epstein-Barr virus, and abnormal liver function test in transfused patients with hereditary hemorrhagic disease. Transfusion 1979; 19:32-8.
43. Hruby MA, Schauf V. Transfusion-related short incubation hepatitis in hemophiliac patients. JAMA 1978; 240:1355-7.
44. Schimpf KL, Zimmermann K, Rudel J, Thanner G, Zeltsch P. Result of liver biopsies, rate of icteric hepatitis and frequency of anti Hb's and Hb's-antigen in patients of the Heidelberg hemophilia centre. Thromb Haemostas 1977; 38:340.
45. Spero JA, Lewis JH, David H, Van Thiel DH, Hasiba U, Rubin BS. Asymptomatic structural liver disease in hemophilia. N Eng J Med 1978; 298:1373-8.
46. Gerstoft J, Dickmeiss E, Bentsen K, et al. The prognosis of asymptomatic homosexual men with decreases T-helper to T-suppreasor ratio. Scand J Immunol 1984; 19:275-80.
47. Fauci AS, Macher AM, Longo DL, et al. Acquired immunodeficiency syndrome. Epidemiologic, clinical, immunologic and therapeutic considerations. Ann Intern Med 1984; 100:92.
48. Gottlieb MS, Groopman JE, Weinstein WM, Fahey JL, Detels R. The acquired immunodeficiency syndrome. Ann Intern Med 1983; 99:208-20.
49. Moll B, Emeson EE, Small CB, Friedland GH, Klein RS, Spigland I. Inverted ratio of inducer to suppressor T-lymphocyte subsets in drug abusers with opportunistic infections. Clin Immunol Immunopathol 1982; 25:417.
50. Metroka CE, Cunningham-Rundles S, Pollack MS, et al. Generalized lymphadenopathy in homosexual men. Ann Intern Med 1983; 99:585-91.
51. Mathur-Wagh U, Enlow RW, Spigland I, et al. Longitudinal study of persistent generalized lymphadenopathy in homosexual men: Relationship to acquired immunodeficiency syndrome. Lancet 1984; 1:1033-8.
52. Abrams DI, Lewis BJ, Volberding PA. Lymphadenopathy: endpoint or prodrome? Ann New York Academ Scien 1984; 437:207-12.
53. Byrum RD, William DC, D'Onotrio S. Risk factors for Kaposi's sarcoma in homosexual men. Lancet 1982; i: 1083-7.
54. Drew WL, Mintz L, Miner RC, Sands M, Ketterer B. Prevalence of cytomegalovirus infection in homosexual men. J Infect Dis 1981; 143:188-92.
55. Lang DJ, Kummer JF. Cytomegalovirus in semen: observations in selected populations. J Infect Dis 1975; 132:472-3.
56. Roux-Lombard P, Aladjem D, Balavoine JF, et al. Lymphadenopathies persistantes generalisees chez les homosexuals et les toxicomanes: alterations de l'immunite humorale et cellulaire. Schweiz. Med Wochenschr 1986; 116:873-80.
57. Mintz L, Drew WL, Miner RC, Braff EH. Cytomegalovirus infections in homosexual men. Ann Int Med 1983; 99:326-9.
58. Rogers MF, Morens DM, Stewart JA, et al. National casecontrol study of Kaposi's sarcoma and Pneumocystis carinii pneumonia in homosexual men : Part 2 : Laboratory results. Ann Int Med 1983; 99:151-8.
59. Fricker HS, Segal S. Narcotic addiction, pregnancy and the newborn. AM J Dis Child 1978; 132:360-6.
60. Ostrea EM, Chavez CJ. Perinatal problems (excluding neonatal withdrawal) in maternal drug addiction: a study of 830 cases. J Pediatr 1979; 94:292-5.
61. Wilson GS, Desmond MM, Wait RB. Follow-up of methadonetreated and untreated narcotic-dependent women and their infants: health, developmental, and social implications. J Pediatr 1981; 98:716-22.
62. Dunn A, Peters RL, Schweitzer IL, Spears RL. Virus-like particles in liver of infants with vertically transmitted hepatitis. Arch Pathol 1972; 94:258-64.
63. Cherubin CE, Rosenthal WS, Stenger RE, et al. Chronic liver disease in asymptomatic narcotic addicts. Ann Inter Med 1972; 76:391.
64. Shiraki K, Yoshthara N, Sakurai M, Eto T, Kawana T. Acute hepatitis B in infants born to carrier mothers with the antibody to hepatitis B antigen. J Pediatr 1980; 97:768-72.
65. Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771-4.
66. Reynolds DW, Stagno S, Alford CA. Antibody response to live virus vaccines in congenital and neonatal cytomegalovirus infections. J Pediatr 1978; 92:738-42.
67. Goldberg GN, Fulginiti VA, Ray CG, et al. In utero Epstein-Barr virus infection. JAMA 1981; 246:1579-81.
68. Kreiss JK, Koech D, Plummer FA, et al. AIDS virus infection in Nairobi prostitute: spread of the epidemic to East Africa. N Engl J Med 1986; 314:414-8.
69. Fishl MA, Dickinson GM, Landesman SH, Friedman SM, Curran JW. Risk factor for AIDS among Haitians residing in the United States. JAMA 1987; 257(5):635-9.
70. Lyons SF, Schoub BD, McGilliuray GM, Sher R, DOB Santos L. Lack of evidence of HTLV-III endemicity in Southern Africa. Lancet 1985; 1(1):1257-8.
71. Bayley AC, Downing RG, Cheingsong-Popov R, Tedder RS, Dalgeish AG, Weiss RA. HTLV-III serology distinguishes atypical and endemic Kaposi's sarcoma in Africa. Lancet 1985; 1:359-61.
72. Pape JW, Liautaud B, Thomas F, et al. The acquired immunodeficiency in Haiti. Ann Inter Med 1985; 103:674-8.
73. Biggar RJ, Gigase PL, Melbye M, et al. Elisa HTLV retrovirus antibody reactivity associated with malaria and immune complexes in healthy Africans. Lancet 1985; 2:520-3.
74. Mann JM, Francis H, Quinn T, et al. Surveillance for AIDS in a Central African City; Kinshasa , Zaire. JAMA 1986; 255(23):3255-9.
75. Nzilambi N, Mann JM, Francis H, Masaki MN, Dikilu K, Malonga M. LAV/HTLV-III seroprevalence among tuberculosis patients in Zaire. Submitted to the International Conference on AIDS, Paris June 23-25, 1986.
76. Clumeck N, Sonnet J, Taelman H, et al. Acquired immunodeficiency syndrome in african patients. N Eng J Med 1984; 210(8):492-7.
77. Colebunders R, Francis H, Mann JM, Kapita B, Ndangi K, Piot P. Clinical manifestations in African AIDS patients. Presented at the International Conference on AIDS, Paris June 23-25, 1986.
78. Piot P, Quinn TC, Taelman H, et al. Acquired immunodeficiency syndrome in a heterosexual population in Zaire. Lancet, 1984; 2(8394):65-9.
79. Serwadda D, Mugerwa RD, Sewankambo NK, et al. Slim disease: a new disease in Uganda and its association with HTLV-III infection. Lancet 1985; 2(8460):849-52.
80. Van De Perre P, Rouvroy D, Lepage P, et al. Acquired immunodeficiency in Rwanda. Lancet 1984; 2(8394):62-5.
81. Fishl MA, Scott GB. The acquired immunodeficiency among Haitians adults and infants: an update. In: Gallin JI, Fauci A, eds. Acquired immunodeficiency syndrome (AIDS). New York, Raven Press, 1985.
82. Brugo MA, Guffanti A, Guzzetti S, Pedretti D, Stringhetti M, Confalonieri F. Differenza nel comportamento delle popolazioni T-linfocitarie nei dipendenti da eroina e da metadone. Boll Ist Sieroter Milan 1983; 6:517.
83. Mac Donough RJ, Madden JJ, Falek A, et al. Alterations of T and null lymphocyte frequency in the peripheral blood of human opiate addicts: in vivo evidence for opiate receptor sites on T-lymphocytes. J Immunol 1980; 125:2539.
84. Weisman M, Lerner N, Vogel WH, Schnoll S. Quality of street heroin. N Engl J Med 1973; 289:698-9.
85. Vogel WH. Toxicity of drugs and "street " drugs: medical and legal problems. Contemp Drug Probl 1971; 1(25):34.
86. Agnew J. Klismaphilia-A physiological perspective. Am J Psycother 1982; 36:554.
87. Denko JD. Rlismaphilia: Enema as a sexual preference. Am J Psycother 1973; 27:232.
88. Denko JD. Klismaphilia: Amplification of the erotic enema deviance. Am J Psycother 1976; 3:236.
89. Kaplan EH. Enemas as a source of sexual pleasure. Med Aspects Human Sex 1976; 10:3.
90. Schnoll SH. Male-sex organ function Lancet 1975; 293(4):202.
91. The Merck Manual, 15th Ed., 1987; P. 860.
92. The Merck Manual, 15th Ed., 1987; P. 89.
93. The Merck Manual, 15th Ed., 1987; P. 85.
94. The Merck Manual, 15th Ed., 1987; P. 197.
95. The Merck Manual, 15th Ed., 1987; P. 213.
96. The Merck Manual, 15th Ed., 1987; P. 780.
97. The Merck Manual, 15th Ed., 1987; P. 204.
98. The Merck Manual, 15th Ed., 1987; P. 2051.
99. Pollack S, Atisa D, Yaffe G, Katz R, Schechter Y, Tatarsky I. Impaired immune function in hemophilia patients treated exclusively with cryoprecipitate: relation to duration of treatment. Amer J Hematol 1985; 20:1.
100. Carr R, Edmond E, Prescott RJ, et al. Abnormalities of circulating lymphocyte subsets in hemophiliacs in an AIDS-free population. Lancet 1984; 1:1431-4.
101. Froebel RS, Madhok R, Forbes CD, Lennie SE, Lowe GDO, Sturrock RD. Immunological abnormalities in haemophilia: are they caused by American factor VIII concentrate? Br Med J 1983; 287:1091-3.
102. Mavligit GM, Talpaz M, Hsia FT, et al. Chronic immune stimulation by sperm alloantigens. JAMA 1984; 251:237-41.
103. Anderson DJ, Tarter TH. Immunosuppressive effects of mouse seminal plasma components in vivo and in vitro. J Immunol 1982; 128:535-9.
104. James K, Harvey J, Bradbury AW, Hargreave TB, Cullen RT. The effect of seminal plasma on macrophage function-a possible contributory factor in sexually transmitted disease. Aids Res 1983; 1(1):45-57.
105. Lord EM, Sensabaugh GF, Stites DP. Immunosuppressive activity of human seminal plasma I. Inhibition of in vitro lymphocytes activation. J Immunol 1977; 118:1704-11.
106. Prakash C, Lang RW. Studies on immune infertility: a hypothesis on the etiology of immune infertility based on the biological role of seminal plasma immune response inhibitor. Mt Sinai J Med 1980; 47:491-510.
107. Franken DR, Slabber CF. Reproductive Immunology: The inhibitory effect of human seminal plasma from normozoospermic men on in vitro lymphocyte cultures. Andrologia 1981; 13:537-40.
108. Witkin SS, Bongiovanni AM, Ing Ru-Yu , Goldstein M, Wallace J, Sonnabend J. Humoral immune responses in healthy heterosexual, homosexual and vasectomized men and in homosexual men with the acquired immune deficiency syndrome. Aids Res 1983; 1(1):31-44.
109. Scrimshaw NS, Taylor CE, Gordon JE. Interaction of nutrition and infection. WHO Monograph Series No. 57 Geneva, WHO 1968.
110. Reusch GT, Ratz M. Proceedings of Symposium held in Haiti, 1977. Am J Clin Nutr 1978; 31:2035.
111. Mata W . The children of Santa Maria Canque: a prospective field study of health and growth in drug users.. Cambridge, MIT Preas 1978.
112. The Merck Manual, 15th Ed., 113. The Merck Manual, 15th Ed.,
114. The Merck Manual, 15th Ed.,
115. The Merck Manual, 15th Ed.,
116. The Merck Manual, 15th Ed.,
117. The Merck Manual, 15th Ed.,
118. The Merck Manual, 15th Ed.,
119. The Merck Manual, 15th Ed.,
120. The Merck Manual, 15th Ed.,
121. The Merck Manual, 15th Ed.,
122. The Merck Manual, 15th Ed.,
123. The Merck Manual, 15th Ed.,
124. The Merck Manual, 15th Ed.,
125. The Merck Manual, 15th Ed.,
126. Sohn N, Robilotti JG. The Gastroenterol 1977; 67:478.
127. Sonh N, Weinstein MA, Gonchar J. Social injuries of the rectum. AM J Surg 1977; 134:611.
128. Hite S. The Hite Report. MacMillan, New York, 1976.
129. Barone JE, Sohn N, Nealon TF. Perforations and forcing bodies in the rectum: Report of 28 cases. Ann Surg 1977; 184:601.
130. Agnew J. Hazards Associated with Anal Erotic Activity. Archives of Sexual Behavior. 1986; 4:307-314.
131. Szunyogh B. Enema injuries. Am J Proctol 1958; 9:303.
132. Large PG, Mukheiber WJ. Injury to rectum and anal canal by enema syringes. Lancet 1956; 2:596
133. Roland CG, Roger AG. Rectal perforations after enema administration. Can Med Assoc J 1959; 81:815.
134. United Nations University. Protein-Energy requirements under conditions prevailing in developing countries: Current Rnowledge and Research Needs. United Nations Univ Food Nutr Bull (Suppl 1 ) 1979.
135. Torun B, Caballero 8, Flores-Huerta S. United Nations Univ Food Nutr Bull Suppl 1984; 10:216-31.
136. Beisel WR, Sawyer WD, Ryll ED, et al. Metabolic effects on intracellular infections in man. Ann Inter Med 1967; 67:744-69.
137. Beisel WR. Magnitude of the host nutritional responses to infection. AM J Clin Nutr 1977; 30:1236-47.
138. Powanda MC. Changes in body balances of nitrogen and other key nutrients: description and underlying mechanism. Am J Clin Nutr 1977; 30:1254-68.
139. Scrimshaw NS. The effect of infection on nutritional status. Bibl Nutr Dieta 1973; 18:153-64.
140. Arbeter A, Echeverri L, Franco D, Munson D, Velez H, Vitale JJ. Nutrition and infection. Fed Proc 1971; 30:1421.
141. Brown RH, Gilman RH, Rhatun M, Ahmed G. Absorption of macronutrients from a rice-vegetable diet before and after treatment of ascariasis in children. Am J Clin Nutr 1980; 33:1975-82.
142. Reusch GT. Immune responses in parasitic diseases. Rev Infec Dis 1982; 4:751-5.
143. Long CL, Jeevanandam M, Rim BM, Kinney JM. Whole body protein syntesis and catabolism in septic man. Am J Clin Nutr 1977; 30:1340-4.
144. Duff JH, Viidik T, Marchuk JB, et al. Femoral arteriovenous aminoacid differences in septic patients. Surgery 1979; 85:344-8.
145. Hermann VM, Clarke D, Wilmore DW, Moore F. Protein metabolism: effect of disease and altered intake on the stable 15N curve. Surg Forum 1980; 31:92-4.
146. Wilmore DW. The metabolic management of the critically ill. New York , Plenum Medical Book, 1977.
147. Beisel WR. Metabolic response to infection. Ann Rev Med 1975; 26:9-20.
148. Wilmore DW, Goodwin CW, Aulick LH, Powanda MC. Effects of injury and infection on visceral metabolism and circulation. Ann Surg 1980; 192:491-504.
149. LaNoue KF, Mason AD, Daniels JP. The impairment of ylucogenesis by gram negative infection. Metabolism 1968; 17:606-11.
150. McFadzean AJS, Yeung RT. Hypoglycaemia in suppurative pancholanglitis due to clonorchis sinensis. Trans R Soc Trop Med Hyg 1965; 59:179-85.
151. Gallin JI, Kaye D, O'Leary WM. Serum lipids in infection. N Engl J Med 1969; 281:1081-6.
152. The Merck Manual, 15th Ed., 1987; P. 1165.
153. Mata LJ, Kromal RA, Urrutia JJ, Garcia B Effect of infection on food intake and the nutritional state: perspectives as viewed from the village. Am J Clin Nutr 1977; 30:1215-27.
154. Lifshitz F. Infections and undernutrition. Nutrition Reviews 1982; 40:119-26.
155. Tomkins A. Nutritional status and severity of diarrhea! diseases among preschool children in rural Nigeria. Lancet 1981; i:860-2.
156. The Merck Manual, 15th Ed., 1987; P. 823.
157. Mangi RG, Niederman JC, Kelleher JE, Dwyer JM, Evans AS, Kantor FS. Depression of cell-mediated immunity during acute infectious mononucleosis. N Engl J Med 1974; 291:1149-53.
158. Carney WP, Rubin RH, Hoffman RA, Hansen WP, Healey K, Hirsh MS. Analysis of T-lymphocyte subsets in cytomegalovirua mononucleosis. J Immunol 1981; 126:2114-6.
159. Reinherz EL, Cooper MD, Schlossman SF, Rosen FS. Abnormalities of T-cell maturation and regulation in human beings with immunodeficiency disorders. J Clin Invest 1981; 68:699-705.
160. Pattengale PK, Smith RW, Perlin E. T-and B-cells markers of atypical lymphocytes in infectious mononucleosis. N Engl J Med 1974; 291:1145-8.
161. Rinaldo CR, Black PH, Hirsh MS. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis 1977; 136:667-678.
162. Ito K, Nakagawa J, Okimoto Y, Nakano H. Chronic hepatitis-migration inhibition of leucocytes in presence of Australia antigen. New Engl J Med 1972; 286:1005.
163. De Gast GC, Honwen B, Nieweg HO. Specific lymphocyte stimulation by purified, heat-inactivated hepatitis-B antigen. Br Med J 1973; 4:707-9.
164. Frei PC, Erard PH, Zinkernagel R. Cell-mediated immunity to hepatitis-associated antigen (HAA) demostrated by leukocyte migration test during and after acute B-hepatitis. Biomedicine (express) 1973; 19:379-83.
165. Young Laiwah AA, Chauduri AKR, Anderson JR. Lymphocyte transformation and leukocyte migration-inhibition by Australia antigen. Clin Exp Immunol 1973; 15:24-34.
166. Reed WD, Mitchell CG, Eddleston ALOOF, Lee WM, Williams R. Exposure and immunity to hepatitis-B virus in a liver unit. Lancet 1974; 1:581-3.
167. Dudley FJ, Fox RA, Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet 1972; 1:723-6.
168. Irwing GR Jr, Hierholzer WJ Jr, Cimis R, McCollum RW. Delayed hypersensitivity in hepatitis B: clinical correlates of in vitro production of migration inhibition factor. J Infect Dis 1974; 130:580-7.
169. Ibrahim AB, Vyas GN, Perkins HA. Immune response to hepatitis B surface antigen. Infect Immunol 1975; 11:137-41
170. Tong MJ, Wallace AM, Peters RL, Reynolds T. Lymphocyte stimulation in hepatitis B infections. N Engl J Med 1975; 293:318-22.
171. Van EPPB DE, Friersen JA, William RC. Immunological studies of anergic patients. Infect Immun 1974; 10:1003.
172. Miller CL. Immunological assays as a measurement of nutritional status: a review. J Parent Ent Nutr 1978; 2:554.
173. Coovadia HM, Wesley A, Brain P, Henderson LG, Hallett AF, Vos GH. Immunoparesis and outcome in measles. Lancet 1977; 619-21.
174. Kantor FS. Infection, energy and cell-mediated immunity. N Engl J Med 1975; 292:629.
175. Crawford DH, Brickell P, Tidman N, McConnell I, Hoffbrand AV, Janossy G. Increased numbers of cells with suppressor T-cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol 1981; 42:291-7.
176. Schooley RT, Hirsh MS, Colvin RB, et al. Association of herpes virus infection with T-lymphocyte subset alterations, glomerulopathy, and opportunistic infections after renal transplantation. N Eng J Med 1983; 308:307-13.
177. Maddision SE, Eldson-Dew R. Non-specific antibodies in amebiasis. Exp Paristol 1971; 11:90-2.
178. Rubin RH, Carney WP, Schooley RT, et al. The effect of infection on T-lymphocyte subpopulations: a preliminary report. Int J Immunopharmacol 1981; 3:307-12.
179. Thomas HC. T-cell subsets in patients with acute and chronic HBV infection, primary biliary cirrhosis and alcohol induced liver disease. Inter J immunopharmacol 1981; 3:301-5.
180. Sirianni MC, Fiorilli M, Pana A, Pezzella M, Ainti F. In vitro transfer of specific reactivity to Cytomegalovirus and Candida to cord blood leukocytes with dializable leukocyte extracts. Clin Immunol Immunolpathol 1979; 14:300-6.
181. Rook AH, Masur H, Lane C, et al. Interleukin 2 enhances the depressed natural killer and cytomegalovirus specific cytotoxic activities of lymphocytes from patients with the acquired immunodeficiency syndrome. J Clin Invest 1983; 72:398-403.
182. Fiorelli M, Sirianni MC, Ianetti P, Pana A, Divizia M, Ainti F. Cell-mediated immunity in human cytomegalovirus infections. Infect Immun 1982; 35:1162-4.
183. Rinaldo CR, Carney WP, Richter BS, Black PH, Hirsh MS. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. J Infect Dis 1980; 141:488-95.
184. Jensen JR, Jorgensen AS, Thestrup-Pedersen K. Depression of natural killer cell activity by syphilitic serum and immune complexes. Br J Vener Dis 1982; 58:298-301.
185. Bloomfield AL, Mateer JG. Changes in skin sensitiveness to tuberculin during epidemic influenza. Am Rev Tuberc 1919; 3:166-8.
186. Mascia AV, Chick FE, Levy WE. Effect of rubeola on tuberculosis. J Pediatr 1953; 43:294-6.
187. Brody JA, Overfield T, Hammes LM. Depression of the tuberculin reaction by viral vaccines. N Engl J Med 1964; 271:1294-6.
188. Belsey MA. Tuberculosis and varicella infections in children. Am J Dis Child 1967; 113:444-8.
189. Reed WP, Olds JW, Kisch AL. Decreased skin test reactivity associated with influenza. J Infect Dis 1972; 125:398-402.
190. Starr S, Berkovich S. The depression of tuberculin reactivity during chickenpox. Pediatrics 1964; 33:769-72.
191. Salaman MH. Immunodepression by mammalian viruses and plasmodia. Proc R Soc Med 1970; 63:11-5.
192. Rinaldo CR, Stossel TP, Black PH, Hirsh MS. Polymorphonuclear leukocyte function during cytomegalovirus mononucleosis. Clin Immunol Immunopathol 1979; 12:331-4.
193. Levin MJ, Rinaldo CR, Leary PL, Zaia JA, Hirsh MS. Immuno response to herpes virus antigens in adults with acute cytomegalovirus mononucleosis. J Infect Dis 1979; 140:851-7
194. Carney WP, Hirsch MS. Mechanisms of immunosuppression in cytomegalovirus mononucleosis II. Virus-monocyte interactions. J Infect Dis 1981; 144:47-54.
195. Carney WP, Iacovello VR, Hirsch MS, Starr SE, Fleisher G, Plotkin SA. T-lymphocyte subsets and lymphocyte response following immunization with cytomegalovirus vaccine. J Infect Dis 1983; 147:958.
196. Dent PB. Immunodepression by oncogenic viruses. Proq Med Virol 1972; 14:1-35.
197. Woodruff JF, Woodruff JJ. The effect of viral infections on the function of the immune system. In: AL Notkins, ed. Viral immunology and immunopathology. Academic Presa, New York 1975:393-418.
198. Osborn JE, Blazcovec AA, Walker DL. Immunosuppression during acute murine cytomegalovirus infection. J Immunol 1968; 100:835-44.
199. Howard RJ, Najaran JS. Cytomegalovirus-induced immune suppression I. Humoral immunity . Clin Exp Immunol 1974; 18:109-18.
200. Booss J, Wheelock EF. Correlation of survival from murine cytomegalovirus infection with spleen cell responsiveness to concanavalin A. Proc Soc Exp Biol Med 1975; 149:443-6.
201. Howard RJ, Miller J, Najarian JS. Cytomegalovirusinduced immune suppression II. Cell-mediated immunity. Clin Exp Immunol 1974; 18:119-26.
202. Selgrade MX, Ahmed A, Sell KW, Gershwin ME, Steinberg AD. Effect of murine cytomegalovirus on the in vitro response of T-and B-cells to mitogens. J Immunol 1976; 116:1459-65.
203. Rubin RH, Cosimi AB, Tolkoff-Rubin NE, Russell PS, Hirsh MS. Infectious disease syndromes due to cytomegalovirus and their significance in renal transplant recipients. Transplantation 1977; 6:458-64.
204. Fiala M, Chatterjee SN, Stacey B. The relationship of cytomegalovirus infection and severe bacterial and fungal infections in renal transplant patients (abstract no. 130). In: Program and abstracts of the 16th Intersciencies Conference on Antimicrobial Agents and Chemotherapy, Chicago, Illinois, Octuber 27-29, 1976. American Society for Microbiology , Washington DC, 1976.
205. Zweiman B, Pappagianis D, Mailbach H, Hildreth EA. Effect of measles immunization on tuberculin hypersensitivity and in vitro lymphocyte reactivity. Int Arch Allergy Appl Immunol 1971; 40:834-41.
206. Finkel A, Dent PB. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol 1973; 6:41-8.
207. Olson GB, Dent PB, Rawls WE. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med 1968; 128:47-68.
208. McMorrow LE, Vesikari T, Wolman SR, Giles GP, Cooper LZ. Suppression of the response of lymphocytes to phytohemaglutinin in rubella. J Infect Dis 1974; 130:464-9
209. South MA, Montgomery JR, Rawls WE. Immune deficiency in congenital rubella and other viral infections. In: D. Bergsma, ed. Immunodeficiency in man and animals. Birth defects: original article series, XI No. 1 Sinauer Associates, Sunderland, Mass 1975:234-8.
210. Pidot ALR, Maurer LH, McIntyre OR. Leukocyte interferon production, RNA synthesis, and PHA response in patients with infectious mononucleosis. Blood 1973; 42:175-85.
211. Twomey JJ. Abnormalities in the mixed leukocyte reaction during iiEectious mononucleosis. J Immunol 1974; 112:2278-81.
212. Chandra RK. Nutrition, inmunity, and infection: present knowledge and future directions. Lancet 1983; 1:688-91.
213. Chandra RK, Gupta S, Singh H. Inducer and suppressor Tcell subsets in protein-energy malnutrition: analysis by monoclonal antibodies. Nutr Res 1982; 2:21-6.
214. Smythe PM, Schonland M, Brereton-Stiles GG, et al. hymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet 1971; ii: 939-43.
215. Chandra RK, Joshi P, Au B, Woodford G, Chandra S. Nutrition and immunocompetence of the elderly, effect of short term nutritional supplementation on cell-mediated immunity and lymphocyte subsets. Nutr Res 1982; 2:223-32.
216. Ferguson AS, Lawlor GJ, Neumann CG, Oh W, Stiahm R. Decreased rosette-forming lymphocytes in malnutrition and intrauterine growth retardation. J Pediatr 1974; 85:717. 217. Chandra RK, Newberne PM. Nutrition, immunity and infection. New York, Plenum Press, 1977.
218. Watson M , Petro TM. C.R.C. Crit. Rev. Microbiol 1984; 10:297.
219. Neumann CG, Lawlor GJ, Stiahm ER, et al. Immunologic responses in malnourished children. Am J Cli Nutr 1975; 28:89.
220. McMurray DN, Loomis SA, Casazza LJ, Rey H, Miranda R. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr 1981; 34:68-77.
221. Bistrian BR, Blackburn GL, Scrimshaw NS, Flatt JP. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr 1975; 28:1148.
222. Chandra RK. Lymphocyte subpopulations in human malnutrition: cytotoxis and suppressor cells. Pediatrics 1977; 59:423.
223. Salimonu LS, Johnson AO, Williams AI, Adeleye GI, Osunkoya BO. The occurence and properties of E rosettes inhibitory substance in the sera of malnourished children. Clin. Immunol Immunopathol 1982; 24:1-7.
224. Schlesinger L, Ohlbaum A, Grez L, Stekel A. Decreased interferon production by leukocytes in marasmus. Am J Clin Nutr 1976; 29:758.
225. Palmblad J, Cantell K, Holm G, Norberg R, Strander H, Sunblad L. Acute energy deprivation in man: effect on serum immunoglobulines, antibody response complement factors 3 and 4 acute phage reactants and interferonproducing capacity of lymphocytes. Clin Exp Immunol 1977; 30:50.
226. Haller L, Zubler RH, Lambert PH. Plasma levels of complement components and complement hemolytic activity in protein-energy malnutrition. Clin Exp Immunol 1978; 34:248.
227. Seth V, Chandra RK. Opsonic activity, phagocytosis and intracellular bactericidal capacity of polymorphs in undernutrition. Arch Dis Child 1972; 47:282.
228. Baumgartner RN, Pollit E. The Bacon Chow study. Analyses of the effects of infectious illness on growth of infants. Nutr Res 1981; 3:9-21.
229. Chandra RK. Fetal malnutrition and postnatal immunocompetence. Am J Dis Child 1975; 129:450-4.
230. Dutz W, Rossipal E, Ghavami H, Vessal K, Kohout E, Post C. Persistent cell-mediated immune-deficiency following infantile stress during the first 6 months of life. Eur J Pediatr 1976; 122:117-30.
231. Chandra RK, Ali SK, Kutty KM, Chandra S. Thymus-dependent lymphocytes and delayed hypersensitivity in low birth weight infants. Biol Neonate 1977; 31:15-8.
232. Ferguson AC. Prolonged impairment of cellular immunity in children with intrauterine growth retardation. J Pediatr 1978; 93:52-6.
233. Chandra RK. Immunocompetence in low-birth-weight infants after intrauterine malnutrition. Lancet 1974; 2:1393.
234. Chandra RK. Cell-mediated immunity in fetally and postnatally malnourished children from India and Newfoundland. In: Malnutrition and the immune response. Suskind RM, ed, New York, Raven Press 1977.
235. Axelrod AK, Trakatellis AC. Relationship of pyridoxine to immunological phenomena. Vitam Horm 1964; 22:591.
236. Ludovici PP, Axelrod AK. Circulating antibodies in Vitamin-deficiency states in pteroylglutamicacid, niacine, tryptophan, vitamin B12, A, and D deficiencies. Proc Soc Exp Biol Med 1951; 77:526.
237. Axelrod AK. Immune processes in vitamin deficiency states. Am J Cli Nutr 1971; 24:265.
238. Pruzansky J, Axelrod AK. Antibody production to diphtheria toxoid in vitamin deficiency state. Proc Soc Exp Biol Med 1955; 89:323.
239. Krishnan S, Bhuyan UN, Talwar GP, Ramalingaswami V. Effect of vitamin A and protein-calorie undernutrition on immune responses. Immunology 1974; 27:383.
240. Nauss KM, Mark DA, Suskind RM. The effect of vitamin A deficiency on the in vitro cellular immune response of rats. J Nutr 1979; 109:1815.
241. Harmon BG, Miller ER, Hoeffer JA, Ullrey DE, Luecke RW. Relationship of specific nutrient deficiencies to antibody production in swine. J Nutr 1963; 79:269-75.
242. Stoerk HC, Eisen HN. Suppression of circulating antibodies in pyridoxine deficiency. Proc Soc Exp Biol Med 1946; 62:88.
243. Dreizen S. Nutrition and the immune response: a review. Int J Vitam Nutr Res 1978; 49:220.
244. Beisel WR. Malnutrition and the immune response. In: Biochemestry of Nutrition I. Neuberger A, Jukes TH, eds, Baltimore, University Park Press 1979;
245. Edelman R. Cell-mediated immune response in proteincalories malnutrition: a review. In: Malnutrition and the immune response. Suskind RM, ed, New York, Raven Press 1977.
246. Fernandes G, Nair M, Onoe K, Tanaka T, Floyd R, Good RA. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc Natl Acad Scien USA 1979; 76:457.
247. Fraker PJ, Hass SM, Luacke RW. Effect of zinc deficiency on the immune response of the young adult A/J mouse. J Nutr 1977; 107:1889.
248. Chandra RK. Iron status, immune response and susceptibility to infection. In: Iron metabolism. Kies H, ed. Ciba Foundation symposium 51. Amsterdam, Elsevier /Excerpta Medica/ North Holland 1977.
249. Selvaraj RJ, Bhat KS. Metabolic and bacterial activities of leukocytes in protein-calorie malnutrition. Am J Clin Nutr 1972; 25:166.
250. Naeye RL, Blanc WA, Paul C. Effects of maternal nutrition on the human fetus. Pediatrics 1973; 52:494-503.
251. Miller HC, Hassanein K. Fetal Malnutrition in white newborn infants: Maternal factors. Pediatrics 1973; 52:504-12.
252. Naeye RL, Blanc WA. Maternal nutrition and fetal growth. Pediatr Res 1973; 7:307.
253. Jelliffe DB. Observations on the teaching of nutrition at maternal and child health centers in some tropical communities. J Trop Pediatr 1974; 20:232-8.
254. Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Am J Med Sc 1959; 237:367.
255. Goudsmith J. Malnutrition and concomitant Herpes virus infection as a possible cause of immune deficiency syndrome in Haitian infants. N Eng J Med 1983; 309:554.
256. Baar, HS. Interstitial plasmacellular pneumonia due to Pneumocystis carinti. J Clin Pathol 1955; 8:19-24.
257. Hughes WT, Price RA, Sisko F, et al. Protein-calorie malnutrition: a host determinant for Pneumocystis carinii infection. Am J Dis Child 1974; 128:44-52.
258. Dossetor J, Whittle HC, Greenwood BM. Persistent measles infection in malnourished children. Br Med J 1977; 1:1633-5.
259. Phillips I, Wharton B. Acute bacterial infection in kwashiorkor and marasmus. Br Med J 1968; 1:407.
260. The Merck Manual, 15th Ed., 1987; P. 67-70.
261. The Merck Manual, 15th Ed., 1987; P. 84.
262. Marino AWM, Mancini HWN. Anal eroticism. Surg Clin N Am 1978; 58:513.
263. Bengoa JM. Nutrition, national development and planning. In: Berg A, Scrimshaw NS, Call DL, eds, Cambridge, MIT Press, 1973:103-25.
264. Berg A. The nutrition factor: Its role in national development. Washington, DC, Brookings Institute, 1973.
265. Toro J. Food and nutrition policy in a changing world. In: Mayer J, Dwyer JT, eds, New York, Oxford Press, 1979:33-66.